Report Materials
Why OIG Did This Review
- Medicare Part B covers wound-care products known as skin substitutes when reasonable and necessary for the treatment of an enrollee’s condition.
- For payment purposes, CMS treats skin substitutes like approved prescription biologics and skin substitutes are reimbursed in non-institutional Part B settings at 106 percent of the average sales price (ASP).
- In March 2023, OIG issued a report that identified significant gaps in manufacturer compliance with new ASP reporting requirements for skin substitutes.
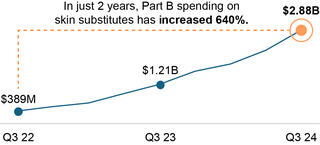
- Despite efforts by CMS to address the accuracy and completeness of ASP reporting, significant increases in expenditures since the OIG report was released raise concerns about what could be driving these trends.
What OIG Found
We identified a number of concerning trends, including the following:
- Medicare Part B expenditures for skin substitutes have skyrocketed over the last 2 years, surpassing $10 billion annually by the end of 2024.
- The rapid growth in expenditures was driven by both increased utilization and higher prices.
- Despite Medicare Advantage having more than half of all Medicare enrollees, utilization and expenditures for skin substitutes under Medicare Advantage were just a fraction of utilization and expenditures under Original Medicare.
- Among enrollees with a skin substitute claim, costs for those reportedly treated at home were four times as high as those treated in an office setting.
- Skin substitutes seem particularly vulnerable to questionable billing and fraud schemes.
Factors that may be driving these trends include:
- Manufacturers’ ability to quickly bring new skin substitutes to the market compared to typical products paid using ASP; and
- Financial incentives such as spread pricing that make certain products more attractive to providers.
Call to Action
Action is urgently needed to rein in the massive increases in Medicare Part B spending for skin substitutes. OIG’s findings illustrate the critical need for payment reforms that address fraud, waste, and abuse in Medicare skin substitute billing. As policymakers consider options, any solutions should ensure that Medicare enrollees continue to receive appropriate care while removing incentives for inappropriate and even fraudulent billing. CMS has recently taken steps toward addressing these concerns.
Notice
This report may be subject to section 5274 of the National Defense Authorization Act Fiscal Year 2023, 117 Pub. L. 263.